Nonwovens For Medical Applications
Innovations in the growing nonwoven medical textiles sector include new products aimed at infection prevention.
Janet Bealer Rodie, Managing Editor
N onwoven textiles play a significant role in the medical sector. The product range includes surgical gowns, masks and other wearable products; surgical drapes, pads; dressings; filtration materials; and implantable textiles such as tissue scaffolds for rebuilding internal organs, among other products.
By far, most nonwoven products used outside the body are disposable, single-use articles that have the advantage of not requiring sterilization or cleaning for reuse. However, there are some that can be reused to provide the required function over a limited period of time.
In North America, disposable nonwoven medical apparel products alone represent a market totaling nearly $1.46 billion, according to the Association of the Nonwoven Fabrics Industry (INDA), Cary, N.C.; and the market is growing by approximately 1 to 2 percent annually. Globally, the medical nonwoven disposables market is forecast to grow to $12 billion annually by 2010, according to market research firm Global Industry Analysts Inc., San Jose, Calif.
Products used inside the body may provide a basis for cells to grow and regenerate tissue — for example, a ligament that has been destroyed or damaged that can be regenerated using a bioabsorbable material that eventually becomes indistinguishable from the ligament itself.
Manufacturing processes used to make these medical nonwovens include needlepunch; hydroentangling; spunbond, meltblown and a combination of the two; and thermal bonding. Bicomponent splittable or fibrillated fibers, nanotechnology and fiber modification also play important roles in some recent developments, a number of them involving filtration and barrier technologies.
“Nanofibers are becoming very popular for medical textiles used to filter viruses and bacteria,” said Jeff Haggard, vice president of technology at West Melbourne, Fla.-based Hills Inc., a developer of man-made fiber technology and machinery. Hills has been a pioneer in the development of bicomponent fibers as well as meltblown and spunbond technologies and their applications, and it offers meltblown technology that can produce fibers in the range between 25 and 400 nanometers (nm), with an average size of 250 nm.
A Collaborative Development
Collaborations between research institutes and private industry have yielded numerous important developments in the nonwoven medical textile field. As one example, Pathogen Removal and Diagnostic Technologies Inc. (PRDT), a joint venture between ProMetic BioSciences Ltd., England, and the American Red Cross, Washington, has developed a filter to remove prion protein from red blood cell concentrate. Prions are responsible for degenerative brain diseases such as mad cow disease and other such diseases, including its human cousin and the target of this filter, variant Creutzfeld-Jakob disease. The filter, marketed in the United Kingdom by France-based MacoPharma S.A. under the brand name P-CAPT™, comprises a target-specific affinity resin sandwiched between nonwoven membranes using a calendering process. The membrane development was carried out through a collaboration with the Nonwovens Cooperative Research Center (NCRC) at North Carolina State University (NCSU). The effort brought together prion experts at the University of Maryland, and chemical engineers involved in bioseparations and NCRC nonwovens staff and engineers at NCSU.
The P-CAPT™ filter for removing prion protein from
red blood cell concentrate comprises a target-specific
affinity resin sandwiched between nonwoven membranes.
Photo and schematic courtesy of MacoPharma S.A.
Disease Prevention: Multiple-Use Protection
Current warnings of a swine flu (H1N1) pandemic must be providing a boon to nonwoven face mask and respirator sales, as people around the world have been shown wearing the masks in an effort to avoid inhaling the virus or spreading possible infection to others. The US Department of Health and Human Services has established a website, www.flu.gov, to provide information about H1N1, avian flu (H5N1) and pandemic flu in general. The website includes a page with information and guidance provided by the Centers for Disease Control and Prevention and the Occupational Safety and Health Administration about the use of masks and respirators to protect against infection. Numerous other websites offered by health organizations and governments around the world also provide relevant information.
Mask manufacturers reportedly are escalating their operations to meet the increased demand. “ We’ve gotten some big orders in several countries and are ramping up production,” said John Dolan, CEO, Carey International Ltd., a Westerly, R.I.-based worldwide distributor of a new, multiple-use respirator mask made with a needlepunched, four-ply fabric comprising two outer layers featuring Agion® silver/copper zeolite compounds permanently embedded into the fiber and two inner filtration layers to prevent microbial or other particle penetration. The outer layers have been shown to kill Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus and other bacteria; and inactivate H1N1, H5N1, the common cold and other viruses. The filtration layers comply with National Institute of Occupational Safety and Health N95 and N99 standards.
The N99 mask — certified according to European Respiratory Protection Standard EN149:2001 FFP3 level to have 99-percent-or-greater particle filtration effcacy, and also approved by Canadian regulatory agencies — is currently available outside the United States. It has been shown in trials to be effective for at least 28 days, compared with eight to 12 hours effective use for standard single-use masks; and the cost per day of use of the mask is about one-tenth of the cost of a single-use mask.
Bill Hurst, director of business development at Wakefield, Mass.-based Agion, said the silver and copper work synergistically to provide faster antimicrobial action than silver alone. “ The ionic exchange between the copper and the sulfur that makes up the bacterium cell membrane helps compromise that membrane,” he said.
Conclusion
Products such as the P-CAPT blood filter and the N99 respirator mask are but two innovations being offered in the growing nonwoven medical textile market. New fiber and processing technologies as well as collaborative, multidisciplinary efforts will contribute to ongoing product development and further market growth.


















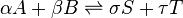
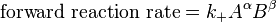





![K_c=\frac{[S]^\sigma [T]^\tau } {[A]^\alpha [B]^\beta}](http://upload.wikimedia.org/math/a/f/6/af61c8307fbde1f9595004610ce90563.png)