
Dry cells do not have a fluid electrolyte. Instead, they use a moist electrolyte paste. Leclanché's cell is a good example of this, where the anode is a zinc container surrounded by a thin layer of manganese dioxide and a moist electrolyte paste of ammonium chloride and zinc chloride mixed with starch. The cell's cathode is represented by a carbon bar inserted on the cell's electrolyte, usually placed in the middle.
Leclanché's simplified half reactions are shown below:
The voltage obtained from the zinc-carbon battery is around 1.5 V.


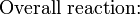
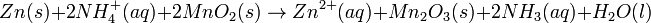

No comments:
Post a Comment